clinical trial supply
The Changing Role of the Data Manager
January 05 by George Clinical RepresentativeThroughout clinical trials, the data manager (DM) is heavily involved in constructing databases, managing reports, programming quality checks and managing the collection of data in the clinical trial....
New Executive Director For The George Institute for Global Health, China
November 10 by George Clinical RepresentativeThe George Institute has recently appointed Professor Zhi-Jie Zheng - a prominent researcher and epidemiologist, as the new Executive Director for its operations in China. For over 30 years, Profess...
Innovation in Clinical Trial Supply
November 09 by Pharmaceuticals & Biotechnology EditorInnovation is thought to be a keystone in the process of being competitive in anymarket. Within clinical trial supply, a lack of innovation can be costly in regards to a range of v...
Clinical Trial Supply 2015 Research
August 17 by Pharma IQ SurveysPharma IQ would like to invite you to take part in this short industry survey. The survey will take less than 10 minutes to complete and as a thank you, all participants who complete the...
The Future of Clinical Trial Supply – Trends and Challenges 2015 Report
October 13 by Pharmaceuticals & Biotechnology EditorIn this report, we gather these responses as well as expert insight on the findings along with interviews with thought leaders and articles on key trends to give you an in depth look at the current st...
Clinical Trial Supply Infographic: Facing Clinical Trial Supply Challenges
October 09 by Pharmaceuticals & Biotechnology Editor45.5% of clinical trial supply professionals consider Brazil to be the most challenging market to work inDid you know that more than 52% of clinical trials professionals express a high interest in lab...
Clinical Trial Supply Infographic: Meeting Challenges with Investment
October 09 by Pharmaceuticals & Biotechnology EditorMore than a quarter of clinical trial supply professionals are looking to invest in outsourcing distribution or logistics Have you ever wanted to know what percentage of professionals ar...
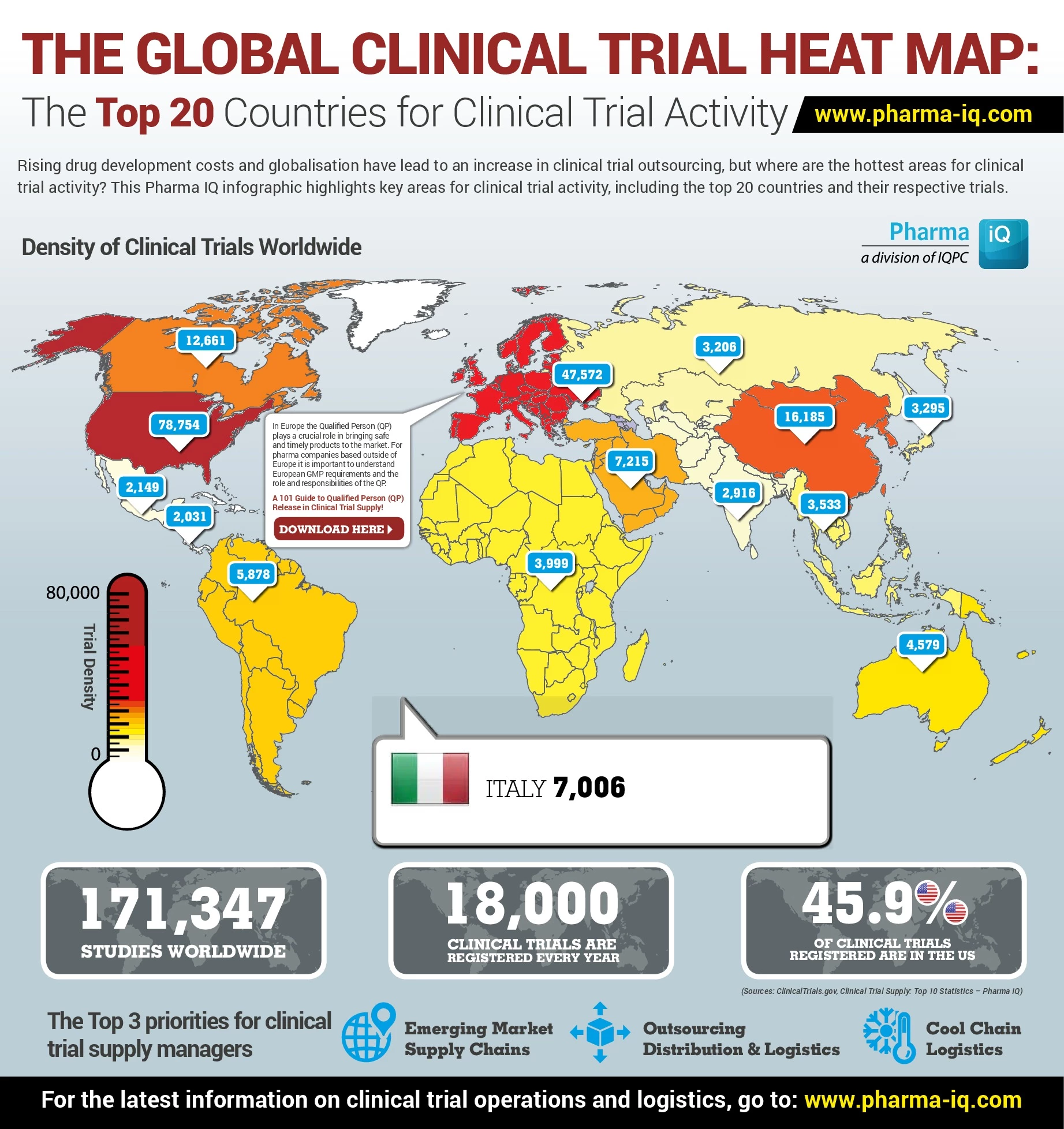
The Global Clinical Trial Heat Map: The Top 20 Countries for Clinical Trial Activity
July 23 by Pharmaceuticals & Biotechnology EditorRising drug development costs and globalisation have lead to an increase in clinical trial outsourcing, but where are the hottest areas for clinical trial activity? This interactive Pharma IQ infogr...
The Trials and Tribulations of Comparator Sourcing for Clinical Trial Materials
June 23 by Pharma IQThe numbers of clinical trials that compare two active drugs, rather than testing a new drug against a placebo, are increasing. Comparator trials are chosen for two main reasons. Firstly, there m...
Clinical Trial Supply Case Study: Bial - An Interview with Ricardo Lima
November 29 by Gerald ClarkeSmaller companies face differing challenges in Clinical Trial Supply, but they also have their own unique advantages. We spoke to Ricardo Lima, Head of Pharmaceutical Development at Bial Resear...
Forecasting a Brighter Future for Clinical Trial Supply in Emerging Markets- An Interview with Adrian Peskett
November 13 by Gerald ClarkePharma IQ interviewed Adrian Peskett, Clinical Supply Logistics Director at Pfizer Ltd. about his predictions for the future of clinical trial supply in emerging markets. Adrian also speaks about th...
Site Perspectives on Clinical Trial Supply- An Interview with Samantha Carmichael
November 05 by Gerald ClarkeShould companies be communicating more clearly with their site teams with regard to their expectations? We spoke to Samantha Carmichael, Lead Pharmacist Clinical Trials at NHS Greater Glasgow &...